Scientists are struggling to develop methods to extract hydrogen from water
Researchers at Lawrence Livermore National Laboratory are continuing to develop the mechanisms they’ve developed to chemically extract hydrogen from water. Hydrogen offers a promising and sustainable carbon-free energy source, and solar-to-fuel technology may be an important factor in its success. The success of solar-to-fuel tech, in turn, depends on whether we can develop efficient, long-lasting and low-cost photoelectrochemical cells (PECs), the tech responsible for absorbing sunlight and driving the reaction that extracts the hydrogen. Such cells have been proven to work, but unfortunately, the technology is in it’s infancy and the PECs remain inefficient.
The laboratoy’s Lawrence Fellow Anh Pham, Assistant Professor Yuan Ping from the University of California at Santa Cruz and Professor Giulia Galli from the University of Chicago and Argonne National Laboratory have been reviewing the interface surfaces between between photoabsorbers, electrolytes and catalysts in PECs in the hopes of finding new ways of improving the cells. A key to developing the technology is finding ideal semiconducting photoelectrode materials which drive the water splitting reaction. Unfortunately, this is a difficult task.
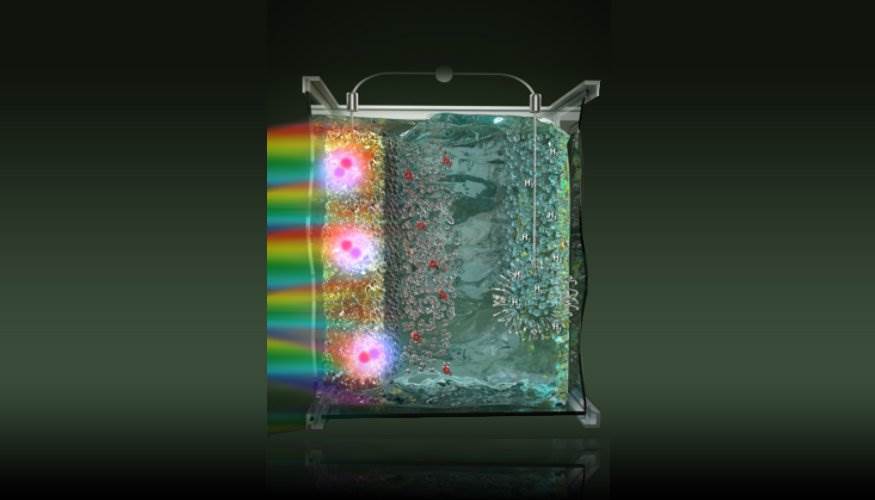
Depiction of the hydrogen extraction in a PEC
“Despite steady efforts and some breakthroughs, no single material has yet been found that simultaneously satisfies the efficiency and stability required for the commercialization of PEC hydrogen production technology,” Pham said. The team’s newly published study discusses how, with the growing complexity of PECs, understanding the properties of its interfaces is key to eventually optimizing performance of the devices.
The team’s approach to the problem involves using a first-principles method – boiling the problem down to its most basic fundamental truths and developing their theories from there. At this point, their first-principles approach is focused on the interplay between the structural and electronic properties of the PEC, and also at the techniques needed to study solid-liquid interfaces, to understand what happens along the surface where the water and PEC meet.
Source: Nature





![[Video] Everyday Freedom, Hands-Free With the Galaxy Buds4](https://loginby.com/itnews/wp-content/uploads/2026/03/Video-Everyday-Freedom-Hands-Free-With-the-Galaxy-Buds4-100x75.jpg)

