“One of the reasons Samsung focused on quantum dots is their exceptionally narrow peaks of the emission spectrum.”
— Sanghyun Sohn, Samsung Electronics
In 2023, the Nobel Prize in Chemistry was awarded for the invention and synthesis of quantum dots. The Nobel Committee acknowledged the groundbreaking achievements of scientists within the discipline — noting that quantum dots have already made vital contributions to the show and medical industries, with broader functions anticipated in electronics, quantum communications and photo voltaic cells.
Quantum dots — ultra-fine semiconductor particles — emit totally different colours of sunshine relying on their measurement, producing exceptionally pure and vivid hues. Samsung Electronics, the world’s main TV producer, has embraced this cutting-edge materials to reinforce show efficiency.
Samsung Newsroom sat down with Taeghwan Hyeon, a distinguished professor within the Department of Chemical and Biological Engineering at Seoul National University (SNU); Doh Chang Lee, a professor within the Department of Chemical and Biomolecular Engineering on the Korea Advanced Institute of Science and Technology (KAIST); and Sanghyun Sohn, Head of Advanced Display Lab, Visual Display (VD) Business at Samsung Electronics, to discover how quantum dots are ushering in a brand new period of show expertise.

Understanding the Band Gap
“To understand quantum dots, one must first grasp the concept of the band gap.”
— Taeghwan Hyeon, Seoul National University
The motion of electrons causes electrical energy. Typically, the outermost electrons — generally known as valence electrons — are concerned on this motion. The vitality vary the place these electrons exist known as the valence band, whereas a better, unoccupied vitality vary that may settle for electrons known as the conduction band.
An electron can take in vitality to leap from the valence band to the conduction band. When the excited electron releases that vitality, it falls again into the valence band. The vitality distinction between these two bands — the quantity of vitality an electron should acquire or lose to maneuver between them — is named the band hole.
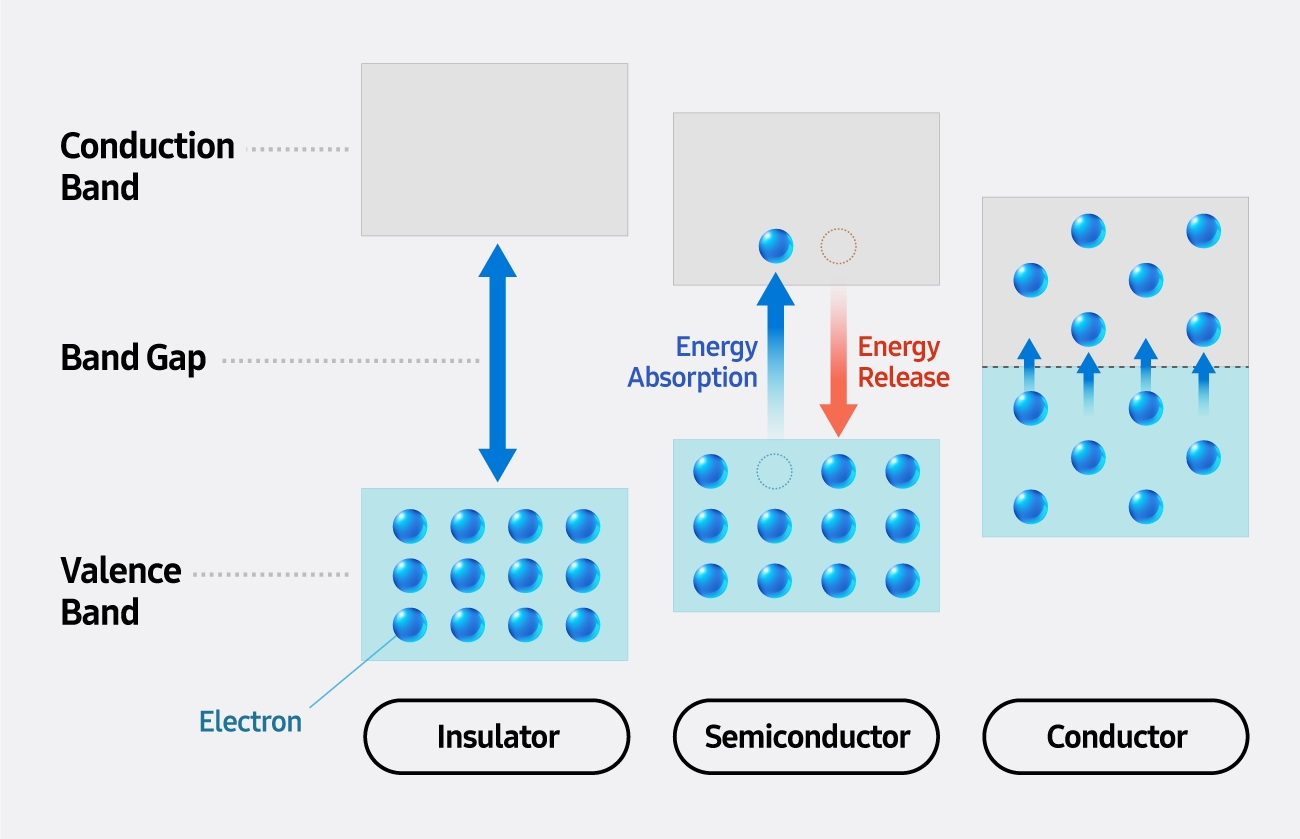
▲ A comparability of vitality band buildings in insulators, semiconductors and conductors
Insulators like rubber and glass have massive band gaps, stopping electrons from transferring freely between bands. In distinction, conductors like copper and silver have overlapping valence and conduction bands — permitting electrons to maneuver freely for top electrical conductivity.
Semiconductors have a band hole that falls between these of insulators and conductors — limiting conductivity underneath regular circumstances however permitting electrical conduction or mild emission when electrons are stimulated by warmth, mild or electrical energy.
“To understand quantum dots, one must first grasp the concept of the band gap,” mentioned Hyeon, emphasizing {that a} materials’s vitality band construction is essential in figuring out its electrical properties.
Quantum Dots – The Smaller the Particle, the Larger the Band Gap
“As quantum dot particles become smaller, the wavelength of emitted light shifts from red to blue.”
— Doh Chang Lee, Korea Advanced Institute of Science and Technology
Quantum dots are nanoscale semiconductor crystals with distinctive electrical and optical properties. Measured in nanometers (nm) — or one-billionth of a meter — these particles are only a few thousandths the thickness of a human hair. When a semiconductor is lowered to the nanometer scale, its properties change considerably in comparison with its bulk state.
In bulk states, particles are sufficiently massive so the electrons within the semiconductor materials can transfer freely with out being constrained by their very own wavelength. This permits vitality ranges — the states that particles occupy when absorbing or releasing vitality — to type a steady spectrum, like an extended slide with a mild slope. In quantum…