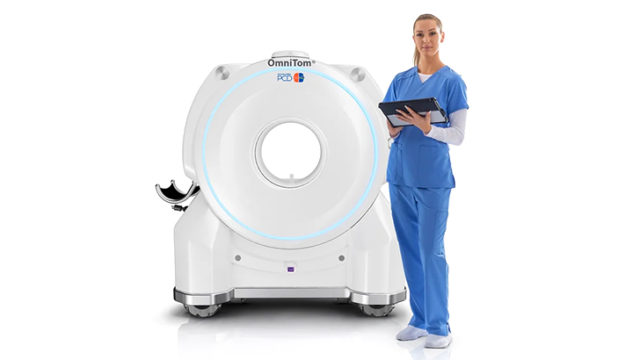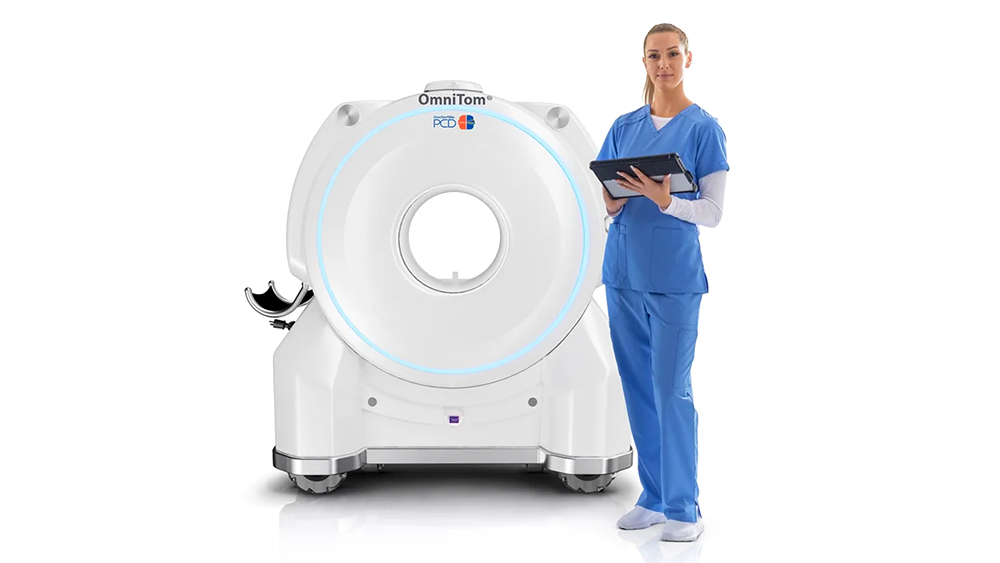
▲ NeuroLogica’s OmniTom® Elite with PCD expertise has acquired U.S. FDA 510(ok) clearance.
NeuroLogica Corp, a subsidiary of Samsung Electronics, introduced its newest configuration of the cellular computed tomography (CT) OmniTom® Elite with Photon Counting Detector (PCD) expertise has acquired U.S. Food and Drug Administration (FDA) 510(ok) clearance. This development builds upon the present PCD capabilities with groundbreaking enhancements, additional solidifying OmniTom® Elite’s place on the forefront of cellular CT imaging expertise.
The newly cleared options optimized for PCD embody a number of important improvements:
- Ultra-High-Resolution Mode: This improvement guarantees unprecedented readability and element in CT imaging. The addition of an ultra-high-resolution mode now presents a formidable 0.141 mm decision.
- Advanced Image Processing Algorithm: The OmniTom® Elite now helps post-reconstruction options comparable to Virtual Non-Contrast (VNC) and bone elimination, enhancing diagnostic capabilities and picture high quality.
- Expanded Scanning Scope: The PCD scanning capabilities now cowl a full 30cm discipline of view, making certain complete imaging potential for a wide range of scientific functions.
- Helical Scanning Capability: This new function permits for steady spiral scanning, offering a quicker and extra environment friendly imaging course of.
- Enhanced Operating Environment: The working atmosphere ambient temperature has been elevated, broadening the vary of circumstances wherein the machine can be utilized.
These enhancements mark a major step ahead from the preliminary PCD configuration, which was already a pioneering achievement in cellular CT expertise. The unique OmniTom® Elite with PCD was the primary FDA 510(ok) cleared, single-source photon counting computed tomography scanner with a single detector, able to producing spectral CT pictures at a number of vitality ranges. This enhancement marks the third 510(ok) clearance for OmniTom® Elite PCD, talking to NeuroLogica’s dedication to steady innovation.
“Samsung and NeuroLogica’s technological innovation has resulted within the FDA clearance of OmniTom® Elite PCD,” stated Kyu Tae Yoo, Head of Samsung Electronics’ Health & Medical Equipment Business and Samsung Medison CEO. “We will continue to develop leading technologies to improve medical staff’s convenience and diagnostic accuracy.”
Renaud Maloberti, Vice President & Head of mCT Business, NeuroLogica, commented, “Our dedication to innovation drives us to repeatedly enhance our merchandise. With these new options, the OmniTom® Elite PCD not solely presents beautiful picture high quality and diagnostic capabilities but in addition ensures a extra versatile and environment friendly imaging course of. This is a major development for point-of-care imaging, persevering with our dedication to allow clinicians to make a extra assured prognosis as a result of improved scientific data.”
The University of Dundee and Massachusetts General Hospital have partnered with NeuroLogica to collaborate on analysis geared toward pushing the boundaries of medical imaging in healthcare utilizing OmniTom® Elite PCD.
Existing OmniTom® Elite prospects can “UpTrade” their scanners to the OmniTom® Elite PCD, granting them entry to the most recent developments in photon counting expertise.
* OmniTom® Elite PCD shouldn’t be presently US FDA 510(ok) cleared for neonate or pediatric scanning and will not be commercially accessible in some international locations. Sales and shipments are efficient solely after approval by the regulatory crew. Please contact your native gross sales consultant for additional particulars. This product is a medical machine; please learn the consumer guide rigorously earlier than use.
About Neurologica
NeuroLogica Corp., the healthcare subsidiary of Samsung Electronics Co., Ltd., develops, manufactures and markets revolutionary imaging applied sciences and is dedicated to delivering quick, simple, and correct diagnostic…